Clinical Safety Assessment
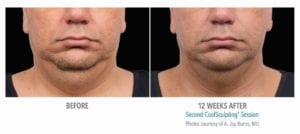
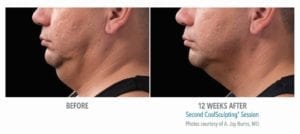
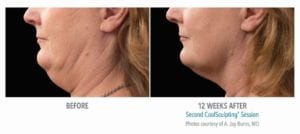
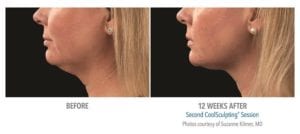
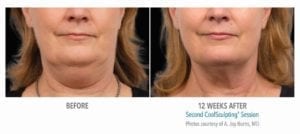
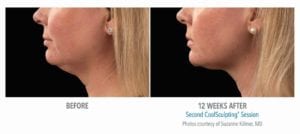
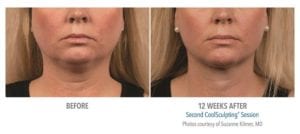
The primary efficacy endpoint was correct identification of pre-treatment vs. 12-week post-final treatment images by 3 blinded independent reviewers. The overall correct identification rate by the 3 reviewers was 91% for the per-protocol population (n=58), which met the pre-established 80% criterion for success. The primary efficacy endpoint was met.
Reduction in subcutaneous fat layer thickness as measured by ultrasound at 12 weeks post-final treatment was a secondary efficacy endpoint for this study. Analysis of the per-protocol data (57 subjects) showed a statistically significant (p<0.0001) reduction of 0.20 cm. Therefore, the secondary efficacy endpoint for the reduction of fat layer thickness was met.
The secondary efficacy endpoint for subject satisfaction was assessed by a questionnaire administered at 12 weeks post-final treatment. Overall, 83.3% of subjects enrolled in the study indicated they were satisfied with the treatment and 80% reported that they would recommend the treatment to a friend.
The primary safety endpoint was the measurement of all device- or procedure-related adverse events. All adverse events reported during and after the treatment were included in the safety analysis. The primary safety endpoint was met. No device- or procedure-related serious adverse events (SAE) and no unanticipated adverse device effects (UADE) occurred during the study. Four device- or procedure-related adverse events were reported and resolved. Clinical safety assessment showed anticipated side effects, all of which were resolved over the course of the study. The safety data recorded during this study supports the safety of the treatment parameters and device investigated.
These clinical findings demonstrate that the use of the CoolSculpting System can safely and effectively affect the appearance of visible fat bulges in the submental area with treatment at -10°C for 60 minutes.